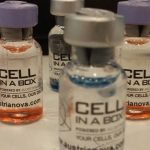
PharmaCyte Biotech (OTCQB: PMCB) has now moved one step closer to submitting an Investigational New Drug application (IND) to the U.S. Food and Drug Administration (FDA) to request a clinical trial in locally advanced, inoperable pancreatic cancer (LAPC) in the United States. The company has successfully completed the first of two manufacturing runs to produce the clinical trial product (Cell-in-a-Box® capsules) it needs for its upcoming Phase 2b clinical trial in LAPC.
Kenneth L. Waggoner, PharmaCyte’s Chief Executive Officer, said that the completion of the first manufacturing run “is a major milestone” towards the completion of the IND.
A few other key events will play themselves out in the weeks to come—all leading to the submission of the IND. The first of which has already begun. PharmaCyte stated this week that the second of two staggered and back-to-back manufacturing runs is already underway, and that the cells from the company’s Master Cell Bank (MCB) are growing well in this second run and will be encapsulated within the next week or two.
A successful second run should be regarded as an even greater milestone and extremely good news for the company and its shareholders as it will represent the conclusion of all necessary manufacturing runs on the way to submitting an IND. cGMP Validation is the company that will take responsibility for PharmaCyte’s clinical trial product coming into the United States and being used in human patients, so two successful manufacturing runs undoubtedly gives cGMP Validation the comfort level it needs to take on this responsibility.
Waggoner said of the second manufacturing run, “Our cGMP expert has recommended that a second manufacturing run be done because, by doing so, we can firmly validate to the FDA that our manufacturing process is both reproducible and robust. Also, additional information on duplicate manufacturing runs may be beneficial to our cGMP expert, who will also serve as our ‘Release Agent’ so that our clinical trial product can be used in human patients in the U.S. in a clinical trial.”
Validating that the company’s manufacturing process is both “reproducible and robust” to the FDA is significant today and well into the future for PharmaCyte, according to the company’s CEO.
“Although we have been advised that the IND for a Phase 2 clinical trial doesn’t require information related to successful duplicate manufacturing runs, it’s important for us to take the extra time to complete the second manufacturing run because the manufactured product is not only the ‘centerpiece’ of our planned clinical trial in LAPC, but it will also likely play a similar role in the treatment of other forms of cancer.”
The most important event left to complete outside of the final manufacturing run is “release testing” of the clinical trial product. PharmaCyte said that a representative sample of frozen syringes from the first successful manufacturing run, which are filled with 300 Cell-in-a-Box® capsules each, are in the process of being shipped to external testing labs for the release testing that is required by the FDA.
The data that those tests will produce are all that remains for the company to complete the IND and then submit it to the FDA.
To learn more about PharmaCyte’s pancreatic cancer treatment and how it works inside the body to treat locally advanced inoperable pancreatic cancer, watch the company’s documentary video complete with medical animations at: https://www.PharmaCyte.com/Cancer
Read our Disclaimer and Disclosure for this company at: https://stockmarketmediagroup.com/disclaimer/